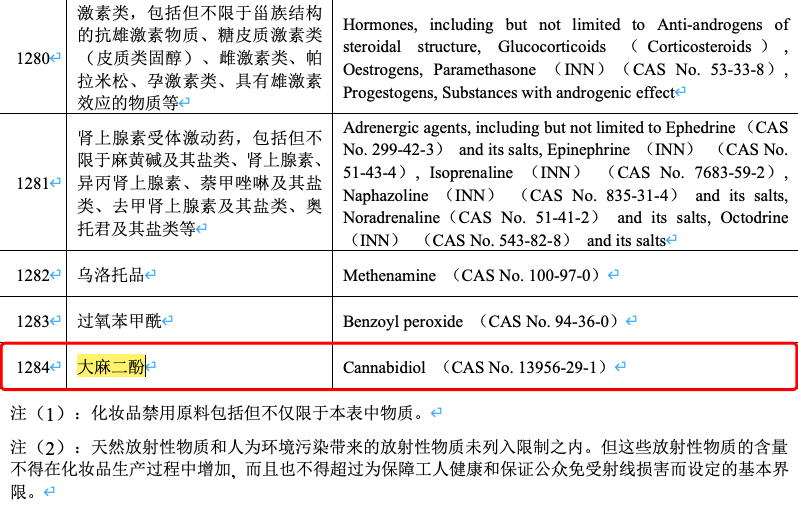
On May 28 the National Medical Products Administration officially updated the catalogue of banned raw materials for cosmetics, representing a final decision on the use of cannabis sativa products. In the future, cannabidiol, cannabis sativa fruit, cannabis sativa seed oil and cannabis sativa leaf extract will be officially on the list of banned raw materials for cosmetics.
In order to further strengthen the management of raw materials for cosmetics and secure the quality and safety of cosmetics, the National Medical Products Administration organized the revision of the Banned Components for Cosmetics (Table 1) and the Banned Plant (Animal) Components for Cosmetics (Table 2) contained in Chapter II of the Technical Specifications for Cosmetics Safety (2015 Edition) pursuant to the Regulations on the Supervision and Administration of Cosmetics, forming the Catalogue of Banned Raw Materials for Cosmetics and the Catalogue of Banned Plant (Animal) Materials for Cosmetics. These were released on May 28 in lieu of the former tables of banned components, respectively, and included in the corresponding chapter of the Specifications.What concerns insiders most is the prohibition of cannabis-related raw materials. The new edition Catalogue of Banned Raw Materials for Cosmetics stipulates the prohibition of the use of Cannabidiol (CAS No. 13956-29-1).The new edition Catalogue of Banned Plant (Animal) Materials for Cosmetics states the ban on the use of CANNABIS SATIVA FRUIT, CANNABIS SATIVA SEED OIL, and CANNABIS SATIVA LEAF EXTRACT.Cannabidiol (also referred to as the CBD component in this area) is a cannabis sativa with tetrahydrocannabinol (THC) content of less than 0.3%. It is a safe non-addictive substance and is among more than one hundred "phytocannabinoids".
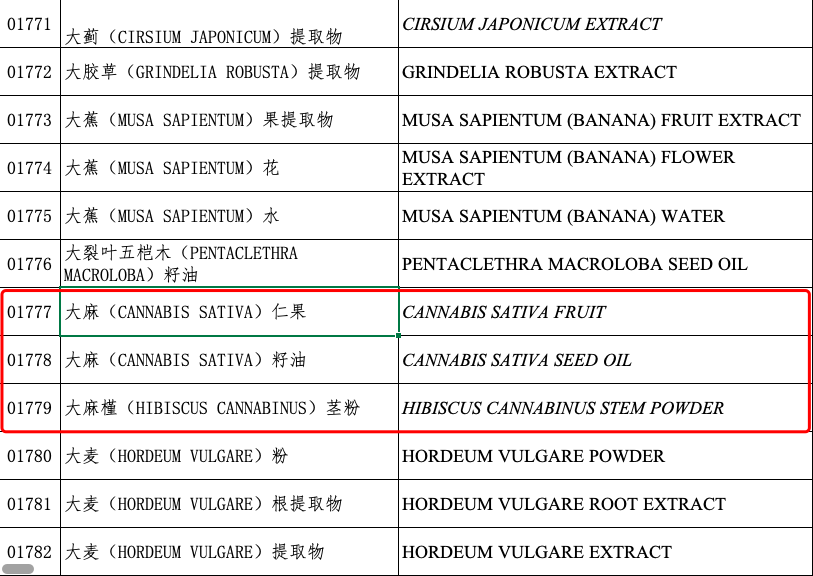
In 2015, the China Food and Drug Administration issued the Catalogue of Names of Raw Materials Used for Cosmetics (2015 Edition), which objectively included the raw materials for the cosmetics produced and marketed in China. The three cannabis-related raw materials, CANNABIS SATIVA FRUIT, CANNABIS SATIVA SEED OIL, and HIBISCUS CANNABINUS STEM POWDER are clearly documented in the Catalogue, further demonstrating the legalization of their application in cosmetics at that time.△ Available cannabis-related raw materials on the Catalogue of Names of Raw Materials Used for Cosmetics (2015 Edition)Given the richness in a variety of biologically active ingredients, including a large amount of terpenoids, polysaccharides, and phenolic substances in addition to cannabinoids, which make CBD anti-inflammatory and antioxidant, CBD has been considered as a "safe" and "legal" industrial cannabis sativa extract by the beauty community.The Circular of the National Institutes for Food and Drug Control on Revising Prohibited Components in Cosmetics for Public Comments published by the National Institutes for Food and Drug Control on March 26 came as a big surprise. It proposed the inclusion of CANNABIS SATIVA FRUIT, CANNABIS SATIVA SEED OIL, CANNABIS SATIVA LEAF EXTRACT, and cannabidiol into prohibited components in cosmetics for public comments, as required, by the relevant policies for national drug control.However, numerous insiders were adopting a 'wait and see' attitude, as domestic cannabis-based cosmetic products boomed at that point.According to statistics, in 2020 alone, 1,783 cannabis-related filings were added to cannabis-based product filings, four times higher compared to 2019. Big brands, such as Origins under Estee Lauder, Avon, and The Body Shop, launched CBD products long early on. In China brands such as Man Ting, Zhi Mei Cun, One Leaf, and HARUKI have all introduced cannabis-based products. Meanwhile, cosmetic brands with CBD as the main ingredient, such as CANNA FEVER New Miracle, Cannabis Cosmetics, and Simpcare, have sprung up in the local market.The introduction of this new regulation completely shattered the domestic cosmetics industry's illusions about cannabis-related products.The National Medical Products Administration also announced that the Circular would take effect on the day of its issuance (May 28).As of the date of the Circular, cosmetics registrants and recorders will be prohibited from manufacturing or importing the cosmetics of which the prohibited raw materials in the Catalogue of Banned Raw Materials for Cosmetics and the Catalogue of Banned Plant (Animal) Materials for Cosmetics are used in the product formulations.CBO will continue to publish in-depth interpretations on the latest Catalogue of Banned Raw Materials for Cosmetics and the Catalogue of Banned Plant (Animal) Materials for Cosmetics.